Sublime
An inspiration engine for ideas

1/30
Our "Protein Project" report is here
What is it?
Unique public-health project funded by @paraschopra to analyze common/well-known protein supplements sold in India
Who did it?
Me & team at The Liver Institute with world class,... See more

Between 2006 and 2019, 9 out of 10 FDA commissioners went on to work for the pharmaceutical companies they were in charge of regulating.
Approximately 65% of the FDA's drug review budget comes directly from the pharmaceutical industry.
Americans are the sickest people on the planet, and our... See more

🚨🚨 Important changes in Health Insurance.
IRDAI released a master circular today.
Most of the changes are applicable from immediate effect - unless there is a specific target date mentioned for the change.
Thread time 👇🏻 https://t.co/79d3CAmoio
"A Johns Hopkins trained physician walked into the Mayo Clinic one night - the emergency department - because he had some known problem that he self-diagnosed. It's very common, it wasn't a big deal, a healthy guy in his 40s, and they said, 'Well, why don't you stay overnight and we'll work you up tomorrow? Then you can drive back home' - because... See more
Jan Jekielekx.com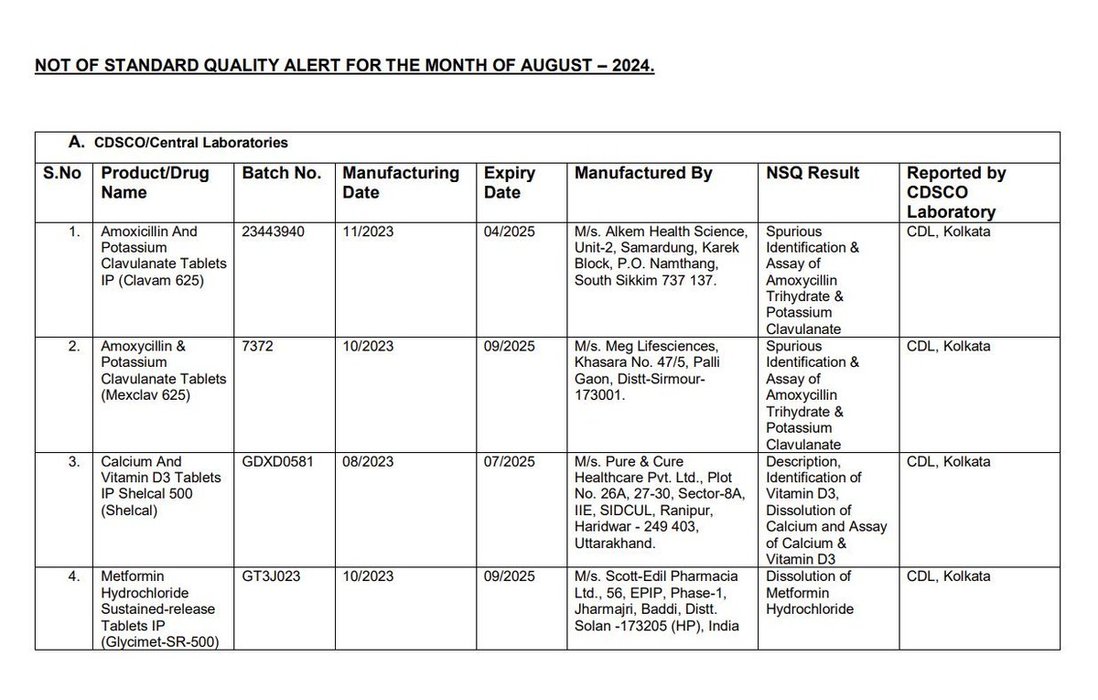
Thread 🧵👇
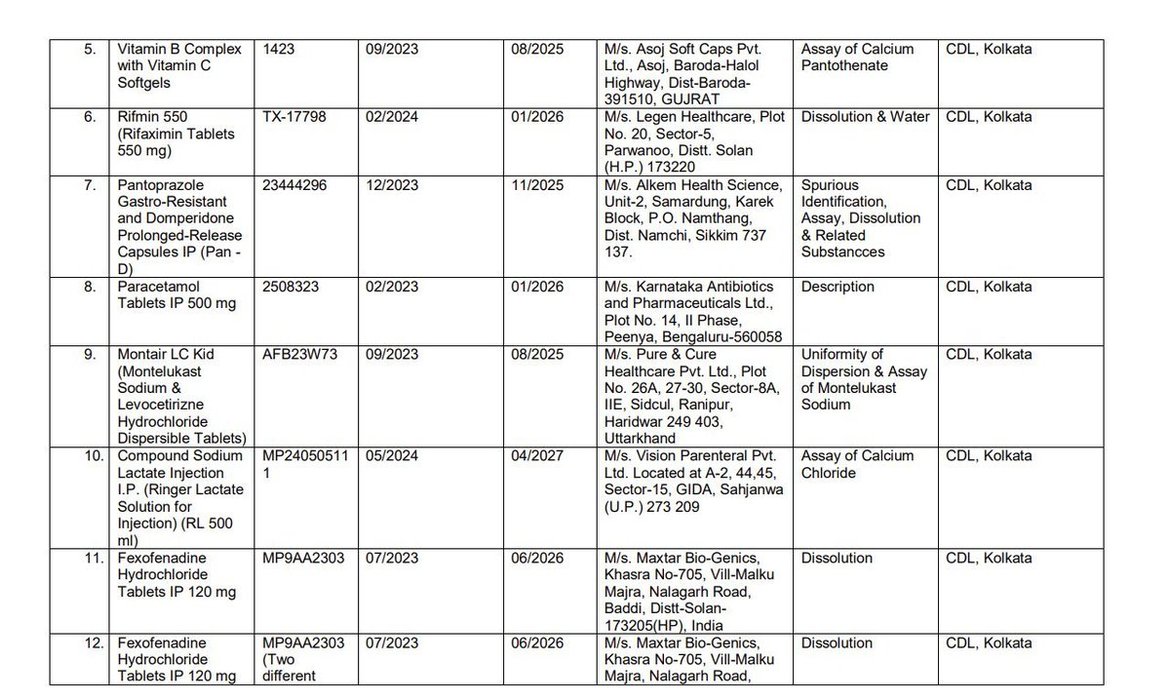
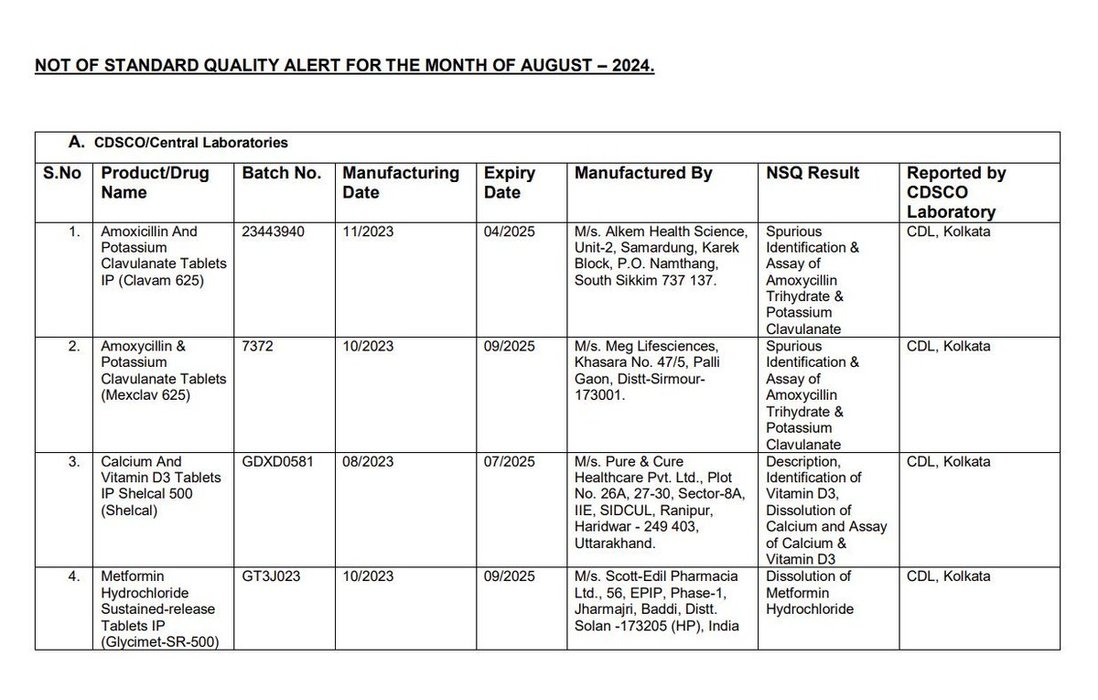
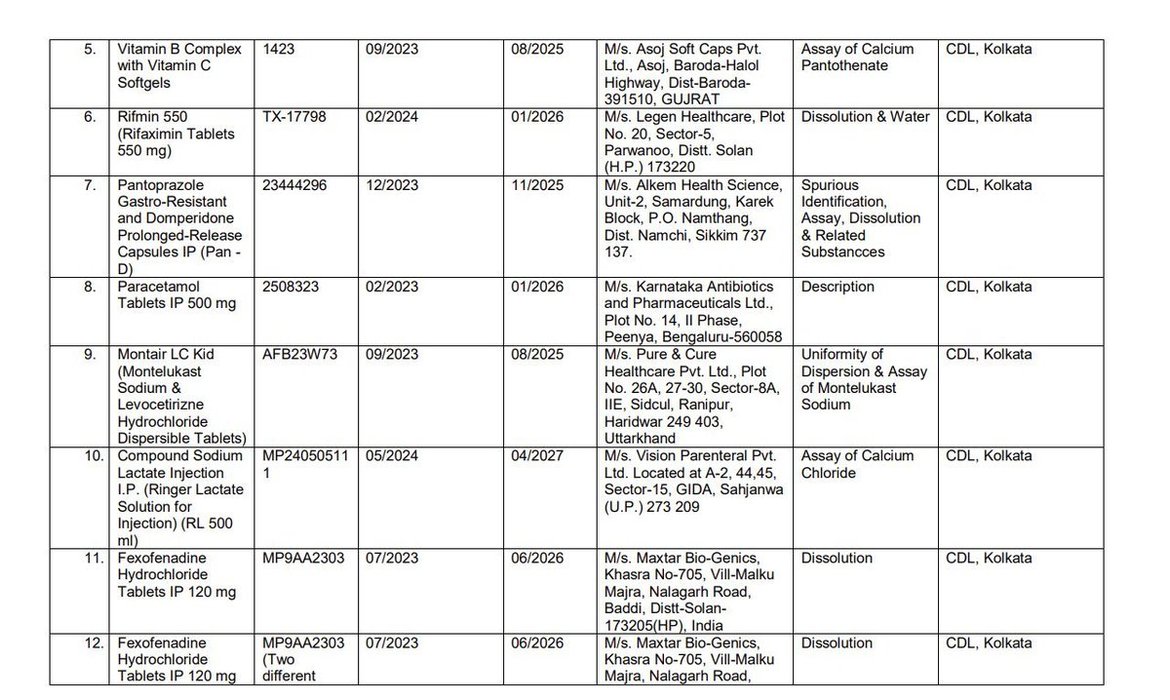
Earlier this week, @CDSCO_INDIA_INF finally relented and published the names of the manufacturers whose products were found to be "Not of Standard Quality" in central and state drug testing laboratories.
There is visible outrage this time, including... See more
Problem statement slide:
We are solving for the four most critical components that are stagnating America’s biopharma innovation engine
1- information asymmetry in the IP generated and what biopharma investors are targeting
2- building a production ready pathway to translate research grade science into commercial grade IP (ai tools fto and experience)
... See moreखाजगी रुग्णालयातील अवाजवी बिलांबाबत तक्रार निवारण समिती- जिल्हाधिकारी राम #पुणे, @Info_Pune @RajSarag दि. 16-
राज्यात कोरोना विषाणू (कोविड१९) चा प्रादुर्भाव रोखण्यासाठी खाजगी हॉस्पीटलमध्ये जादा बिलासंबंधित प्राप्त तक्रारीच्या अनुषंगाने जिल्हा स्तरीय तक्रार निवारण समिती गठीत
collectorpunex.com
Dear @ECISVEEP, hoping that a similar notice, with a similar deadline has also been sent to the @HMOIndia. https://t.co/wa2o5SDVw4
The FDA is sitting on a goldmine of drug development data — 10,000+ page dossiers detailing how drugs are designed, made, tested, and approved.
Big Pharma has these in-house. Startups? Locked out.
It’s time to fix that. Read my proposal for @IFP on how.
https://t.co/rPrLuG7tHz
Ruxandra Teslo 🧬x.com